Nuvaxovid
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre.
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News
Clinical trials showed that the vaccine has around 90 efficacy.

. Beslutet är temporärt och gäller från. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. 16 fever including 14 severe cases.
COVID-19 Vaccine recombinant adjuvanted 2. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Data från Australien pekar mot en ökad.
Nuvaxovid dispersion for injection. First Approval of the Protein-Based Adjuvanted Nuvaxovid NVX-CoV2373 Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30.
88 experienced pain. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over. The Summary of Product Characteristics is a description of a.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. 1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. The addition of the saponin-based.
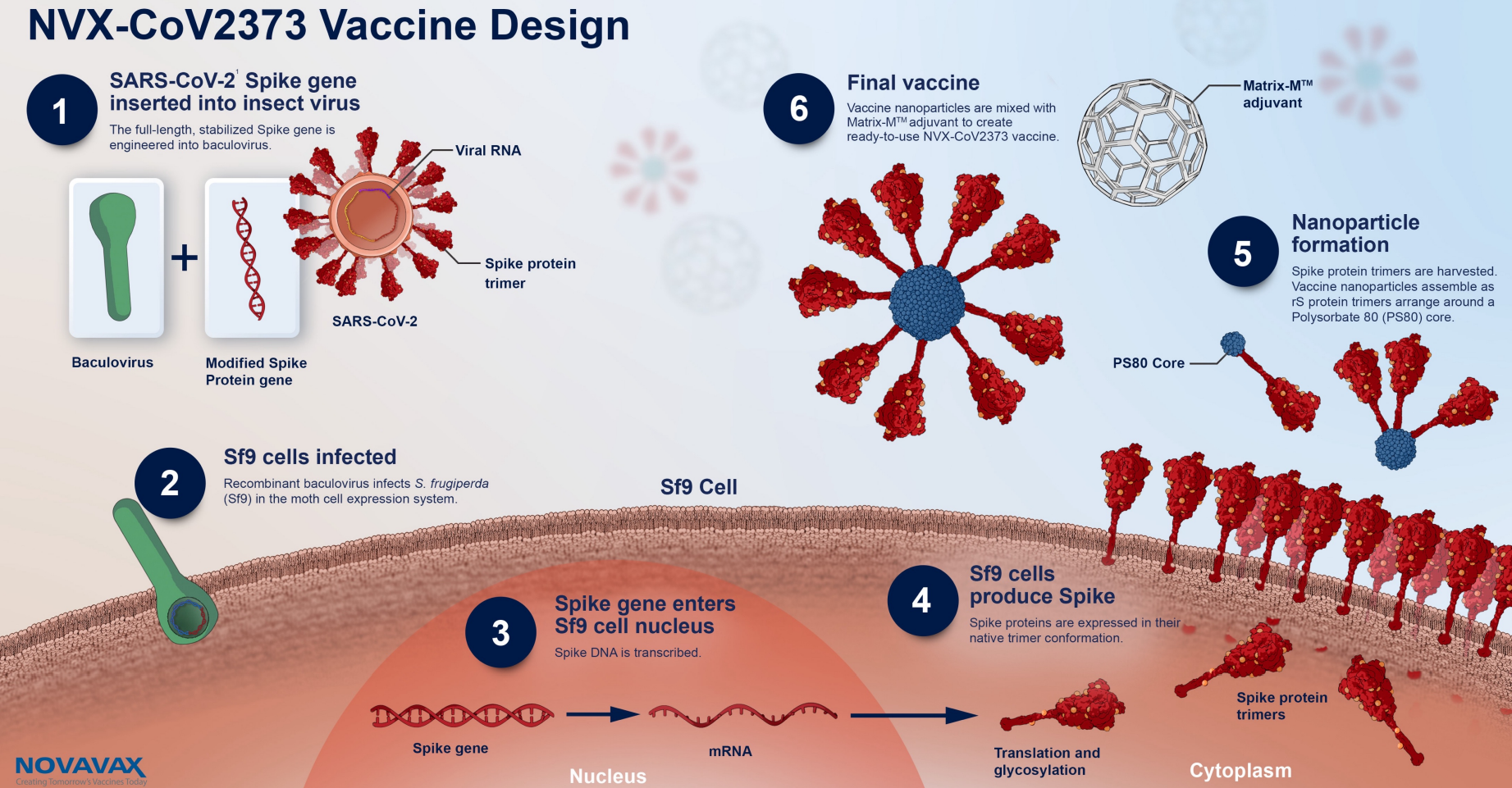
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
Novavax is approved and available for use as a booster in. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency. Qualitative and quantitative composition.
Det eftersom att data från. Rokotteesta ei myöskään ole haittaa vaikka. Vi använder cookies egna och tredje part på denna webbplats för att bland annat personalisera innehåll och annonser i våra tjänster för.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. On December 20 2021 the. Nuvaxovid-rokote sopii lähes kaikille aikuisille.
7 hours agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. 1 day agoVaccinet Nuvaxovid mot covid-19 stoppas. 1 day agoPublicerad idag 0702.
1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Nuvaxovid is the first protein-based COVID-19 vaccine granted. This vaccine is currently being used in Sweden and as of date a total of 7000.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. Name of the medicinal product. This is a multidose vial.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
Like the Novavax vaccine side effects were more.

Novavax Nuvaxovid Covid 19 Vaccines Will Also Be Available From Rauma Healthcare Services In The Future Rauma Fi

Covid 19 Nuvaxovid Novavax Veksin Or Maalumat Australian Government Department Of Health And Aged Care
Racgp Tga Green Lights Nuvaxovid For 12 17 Year Olds
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News
Nuvaxovid Otazky A Odpovedi K Vakcine Od Spolecnosti Novavax Kurzy Cz

European Union Authorizes Novavax Booster
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc

Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022
Coronavirus Who Approves Novavax As 10th Authorised Covid Jab

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary

Informacoes Sobre A Vacina Nuvaxovid Novavax Contra Covid 19 Australian Government Department Of Health And Aged Care

Fda Advisers Back Authorization Of Novavax Covid Shot Medpage Today

Cdc Endorses Novavax Covid Shot For Adults Fortune

Covid Vaccine Novavax Requests Who To Expand Emergency Use Listing Of Nuvaxovid For Adolescents Aged 12
Japan Approves Novavax As 4th Covid Vaccine Amid New Surge Bloomberg



